1st edition, 1932; Wiley
This remarkable scientist was born in 1886; she became a professor just after World War I ended, and worked until five years before her death. Her textbook, “Experimental Cookery: From the Chemical and Physical Standpoint (With a Laboratory Outline)” is certainly the intellectual precursor of better-known books such as McGee’s “On Food and Cooking”.
This is a perfect example, to me, of a smart female scientist being denied access to ‘real’ science, and turning to an acceptably feminine branch instead. The book is incredibly well-researched and referenced; the meticulous experimental protocols leave me yearning to test exactly what effect stirring time does have on muffin shape. She devoted her life to working out the best scientific conditions for cooking, with precision, accuracy, and detail.
Emulsions
Bancroft states that the necessary conditions for forming a stable emulsion are that the drops of the dispersed phase shall be so small that they will stay suspended and that there shall be a sufficiently viscous film around each drop to keep the drops of the dispersed phase from coalescing... Fischer and Hooker state that hydrated colloids make the best emusifiers. For the formation of a stable emulsion the colloid must absorb or bind all the water... The hydration theory... seems to be most applicable to emulsions like cream puffs, in which the emulsion is subjected to heat during cooking, and the presence of too small a quantity of water causes breaking of the emulsion.
…
Seifriz, in his work with petroleum oil emulsions stabilized with casein solution [a milk protein], found that the oils with a specific gravity of 0.828 or below form oil-in-water emulsions… [he] concludes that ‘the chemical interaction of oil, stabilizer, and electrolytes determines the behavior of emulsions, and… is likely to differ if one of the three factors is changed.’
[Mayonnaise]
With egg yolk all the oils and fats [butter, lard, Crisco, Snowdrift; cottonseed, corn, olive, and peanut oils] gave a very stable emulsion of the oil-in-water type. With egg white as the emulsifier, oil forms an oil-in-water emulsion… When the oil is added gradually to the egg white, a foam as well as an emulsion is formed… The illustration also shows that the oil is adsorbed in the film or layer at the interface between a liquid and a gas.
…
With a saturated casein solution as the emulsifier, the water-in-oil type of emulsion predominates when butter, lard, Crisco, and oil are used. The emulsions are rather coarse and unstable. With milk as the emulsifier no permanent emulsion has been obtained with corn or cottonseed oil… When allowed to cool so that the fat solidifies, the emulsions made with fat remain permanent, until stirred. When stirred after the fat has hardened the water collects in large drops, and is similar to collecting of water when butter is worked.
Factors Affecting the Formation of Emulsion in Mayonnaise
(1) The kind of bowl and beating utensil used. (2) The method of mixing. (3) The quantity of oil that is added at first. (4) The kind of oil used. (5) The method of adding the vinegar. (6) The amount of salt, spices, and vinegar used. (7) Use of fresh or storage eggs. (8) The addition of a small amount of emulsified mayonnaise. (9) Temperature.
[highlights]
-Microscopic examination of mayonnaise made by intermittent and continuous mixing shows little difference in the size of the oil particles and the stability of the emulsion.
-Mayonnaise is formed more readily if the quantity of oil added at first is small. Robinson states that, if the oil is added too rapidly… the oil phase may unite, and breaking of the emulsion occurs.
-The following amounts of oil could be emulsified in 15.5 grams of egg yolk:
Pure Italian olive oil.......296 grams
California olive oil..........344 grams
Mazola..............................399 grams
Wesson’s oil....................432 grams
-However, the size of the oil particles that are first emulsified is quite large when the vinegar is added to the egg yolk before ht eoil is added. But with each subsequent addition of oil the dispersed particles become smaller, and the mayonnaise stiffer.
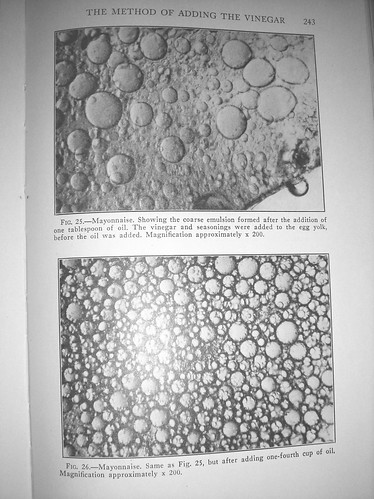
-When the salt is added to the mayonnaise after the oil has been added, the size of the dispersed oil globules is larger and the emulsion breaks more readily...
-Egg yolk, whole egg, egg white, cooked egg yolk, gelatin, and starch pastes may be used for the emulsifying agent in making mayonnaise, if an oil and not a fat is used for the dispersed phase. However, they are not equally efficient. Microscopic examination shows that egg yolk emulsions have the smallest oil globules, gelatin and cornstarch paste the largest...Meat extract and some other materials may be used for emulsifiers, but most of them are not so satisfactory as the egg yolk.